Life cycle assessment of pharmaceutical tablet manufacturing: A comparative analysis and systems model integration framework
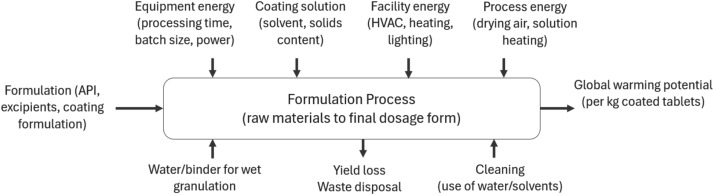
This paper presents an analysis of the sustainability impact of different oral solid dosage manufacturing platforms using life cycle assessment (LCA) methodology. The carbon cost of continuous direct compression (CDC), roller compaction (RC), direct compression (DC) and high shear granulation (HSG) is considered as a function of manufacturing scale, accounting for material, yield losses, process and equipment, cleaning, and utility impacts. The LCA calculations are integrated within a system model for the CDC platform (CDC digital twin), and performance is assessed using feeder variability with different blender residence masses, to demonstrate proof-of-concept for consideration of carbon footprint within process optimisation.
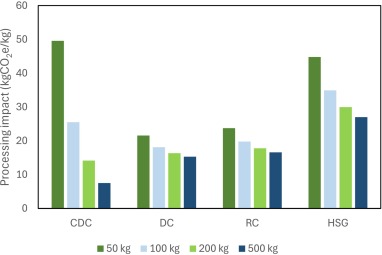
Figure credits: Flora Bouchier, Astrid Boje, Gavin Reynolds, International Journal of Pharmaceutics (2025). doi: 10.1016/j.ijpx.2025.100395.