Investigating the Composition of the Metal Dimer Site in Chabazite for Direct Methane-to-Methanol Conversion
In this paper, we study the complete reaction cycle for methane-to-methanol conversion over various transition-metal dimers
in the chabazite zeolite using density functional theory calculations and first-principles-informed microkinetic modelling,
building on our previous reaction mechanism for the Cu2 dimer under dry and wet (with an additional H2O molecule) conditions.
Using the adsorption energy of atomic oxygen as the descriptor, we establish scaling relations for the energy landscapes and generate the volcano plot for reaction rate.
Both the first-principles-informed microkinetic model and the volcano plot indicate good activity for the Cu2, AuPd, and PdCu systems under technologically relevant conditions.
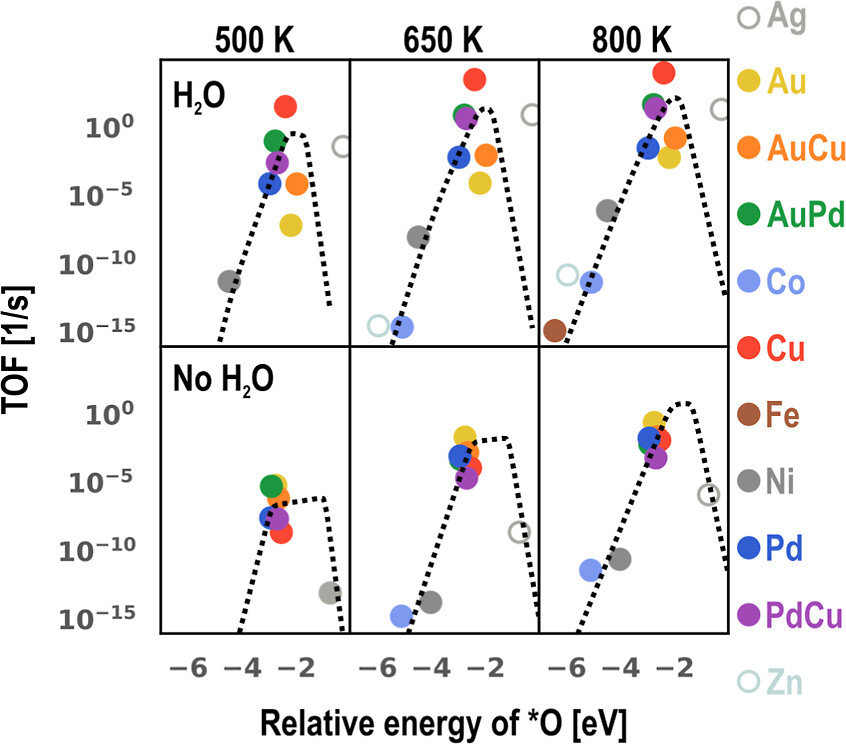
Figure credit: Engedahl, Boje, et al., J. Phys. Chem. C. 2024 (ASAP). doi: 10.1021/acs.jpcc.3c06635.