Complete Reaction Cycle for Methane-to-Methanol Conversion over Cu-SSZ-13: First-Principles Calculations and Microkinetic Modeling
In this paper, we study the complete reaction cycle for methane-to-methanol conversion over Cu-SSZ-13
using density functional theory calculations and first-principles-informed microkinetic modelling.
We study the activity of dry and wet catalyst systems, finding that humidity significantly improves the
turnover frequency, with even low water partial pressures enabling good performance at 450 K.
The predicted apparent activation energy agrees well with experimental data; thus, since the studied mechanism
does not describe the more energetically preferred formation of experimentally observed products such as
carbon dioxide, the results suggest that selectivity, rather than inherent kinetic limitations,
is an important target for improving methanol yield from humid systems.
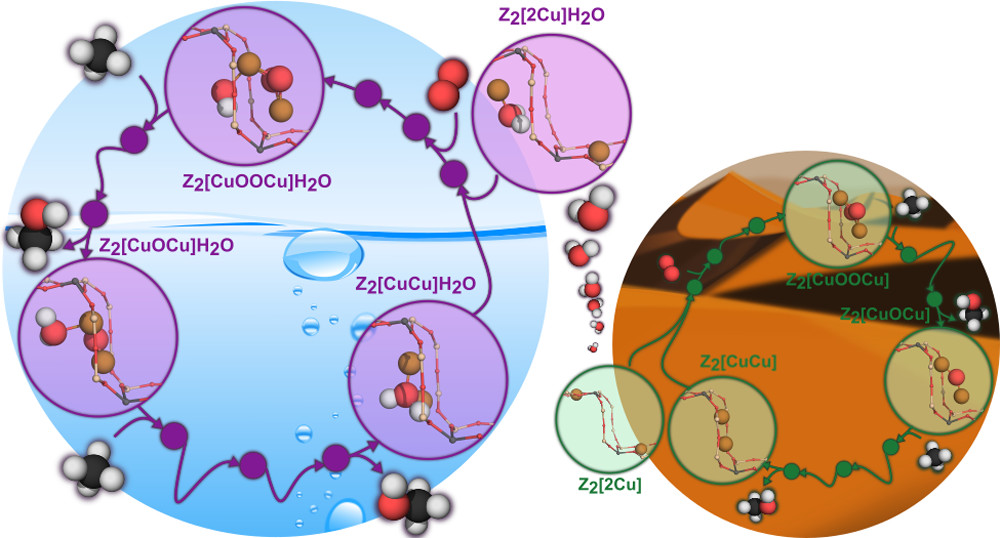
Figure credit: Engedahl, Boje, et al., J. Phys. Chem. C. 2021 (ASAP). doi: 10.1021/acsnano.1c01537.