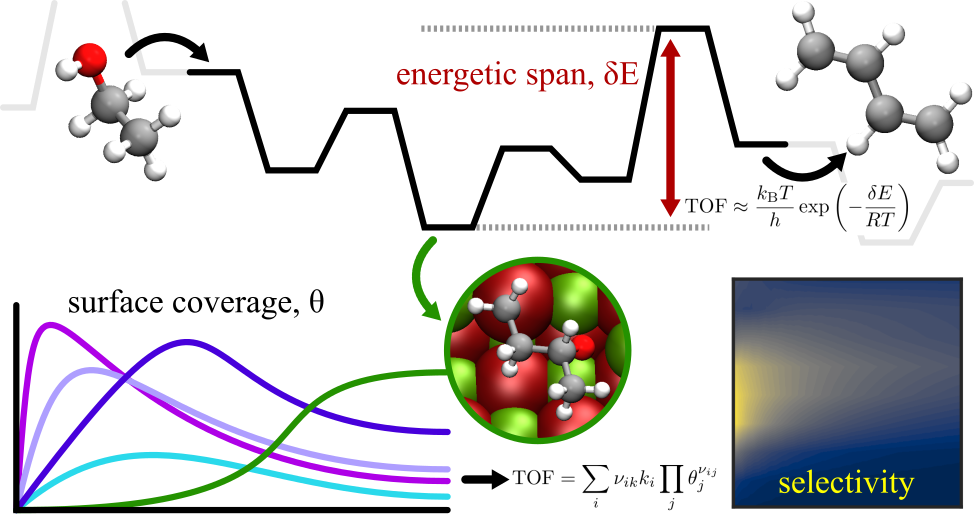
I published a new preprint to ChemRxiv: First-principles-informed energetic span and microkinetic analysis of ethanol catalytic conversion to 1,3-butadiene on MgO. It presents a kinetic study of catalytic conversion of ethanol to 1,3-butadiene, which has applications in polymer synthesis and as an intermediate in organic chemistry. In this paper, we utilized energetic span and microkinetic analysis to study the activity and selectivity of ethanol conversion on an MgO (100) step-edge. These two perspectives provided insight into the most active pathway (dehydrogenation of ethanol followed by C-C bond formation via an aldol condensation mechanism), rate-determining transition states and intermediates such as adsorbed ethanol and four-carbon intermediates in the Prins condensation mechanism, the influence of surface coverage and competing reaction pathways on the turnover, and the selectivity to 1,3-butadiene under a range of process conditions. We considered the non-uniform influence of temperature on the free energy landscape by accounting for contributions to the free energy from translational, vibrational, and rotational degrees of freedom and demonstrated how this leads to differing rate-determining states and dynamics at higher and lower temperatures.